By Bergit Korschan-Kuhle, LHRM-MDS-Pat-IG, Germany, MDS-Alliance
Impressions of the 15 th International MDS-Symposium in Copenhagen in May 2019 – from the patient perspective
Having lived with Myelodysplastic Syndromes (MDS) for more than 13 years now, it seems unbelievable and fascinating to me how much progress has been made since 2005.
In particular, the advances in the diagnosis of MDS achieved by the growing understanding of disease-related pathobiology, molecular genetics, and the processes of cell division and cell metabolism are very promising.
Yet, MDS represent an extremely heterogeneous and dynamic group of diseases. This means there are many subgroups which may behave differently over time, some moving slowly, others behaving more aggressively. More and urgent research is needed to find targeted therapies.
Lesson to learn: most probably there never will be the one and only cure for MDS, but personalized medicine with drugs dependent on the individually impaired genetic profiles.
Valuable insight and increasing knowledge about the origin of MDS: the source of optimism
In Copenhagen, world renowned MDS scientists and clinicians from all over the world presented their highly acclaimed findings in these key areas.
I am not a physician, nor a geneticist or graduated biochemist. All those degrees would enable me to better understand the data presented at conferences about MDS than I currently do. In fact, I have been attending MDS-congresses for many years, but still I need to google many acronyms, and after conferences I usually need to ask experts to translate relevant biochemical mechanisms, processes, and their interrelationships into more lay language. The more the
researchers focus on the microcosm of the cell, including the world of proteins, the building blocks of the DNA and, into molecular signaling pathways, the more complex and complicated it becomes to understand my disease. Amateurish knowledge does not lead me anywhere. I must abandon my inherent principle to base criticism and making demands on a full understanding of the disease. I am aware that I will rant without capturing the full disease landscape. On the other hand, I can rely on a sufficient overview of knowledge to assess the situation for
MDS patients.
At the 15 th International Symposium on MDS in Copenhagen in May 2019 slight optimism was spread by top-level speakers that the valuable insight into, and the increasing knowledge about the origin of the disease, will lead to targeted therapies soon. Speakers expressly emphasized the availability of top qualified young innovative researchers and the fruitful collaboration between researchers and clinicians across the globe. They confirmed there is funding for research and that new regulations for clinical trials will hopefully allow for inclusion of patients
into these trials (e.g. inclusion criteria for patients’ recruitment). Eight drugs have been approved for AML (Acute Myeloid Leukemia) in 2017-2018, which is seen as an encouragement for MDS research and potential drug development.
Time is required to bring a new drug to market and some outcomes are disappointing
However, this optimism must be tempered by understanding the amount of time required to bring a new drug to market where patients not involved in clinical trials have access. We patients know it takes a long way from bench to bedside and most of the MDS patients have only little time to wait for another 10 years. Physicians and patients are impatiently waiting for some major breakthrough, whereas researchers who work hard on different therapy approaches are still struggling with so many questions. Currently there is nothing more than hope. Hope is not a category that scientists report in their work. Rather, they report evidence-based data, response rates of drugs, percentage of overall survival, and patient-relevant endpoints. But there is no new MDS drug to report on.
The European Medicines Agency (EMA) has approved four drugs tackling MDS in one way or the other since 2006. Azacitidine (Aza) for high risk MDS-patients, was approved in 2008: 11 years ago! Besides Aza there is a similar compound called Decitabine, not yet approved by EMA, but by FDA and in EU countries administered in case Aza is failing. Azacitidine has a response rate of 40-47% and statistically an overall survival benefit of nine months. Revlimid (Lenalidomide) is a third option approved in some countries for MDS with del5q. Unfortunately, less than 15% of patients with MDS fit these criteria and if there are additional mutations, particularly TP53, this drug is of limited benefit.
Professor J. P Issa from the Coriell Institute for Medical Research in New Jersey called his talk “What’s after AZA?” Most effectively, he presented a white, empty slide: “Nothing so far”, he confirmed. In fact, he afterwards continued by enumerating several combination-therapies with Aza plus compound x being tested in Phase 1 or 2 clinical studies or located in the pipeline of the pharmaceutical industry. These combination drugs aim to reactivate or enhance Aza at the time of Aza failure. As it has turned out, many of the combination therapies in Phase 1 or 2 clinical trials have increased toxicities with low to moderate response rates. Among these approaches is a combination with AZA and Vitamin C which, in some cases, has shown an increase in effectiveness of Azacitidine.
Prof. David P. Steensma from the Dana-Farber Cancer Institute in Boston, MA, was one of the last speakers of the congress. He named his scientific talk “Bridging the Canyon between Discovery and Therapy”, my column is referring to this title. As an introductory sentence, he asked himself, whether – after Copenhagen – he would treat MDS-patients differently as before? His answer was “No!” He topped that by describing MDS treating techniques and therapies as not having changed for many years. Although the molecular science of the disease has advanced significantly, this has not led to new drug approvals. In addition, he mentioned drowning in bureaucracy and red tape, it usually takes many months to open a full protocol so that patients can participate in a clinical trial. Yet, all currently available drugs are derived from clinical trials and continuing patient enrollment in these trials will be necessary to find new drugs. I don’t have to be a scientist to understand the extent of barriers to hope. One decisive improvement could be to involve patients/patient advocates into research and development. Patients are experts in patients’ needs and could put pressure on drug development in the right and meaningful direction.
Apart from the disappointing outcomes concerning new treatments for MDS patients, it was a great congress. The organization of the MDS Foundation was brilliant and additionally a good platform for networking. I definitely expanded my horizon from the excellent talks of the speakers.







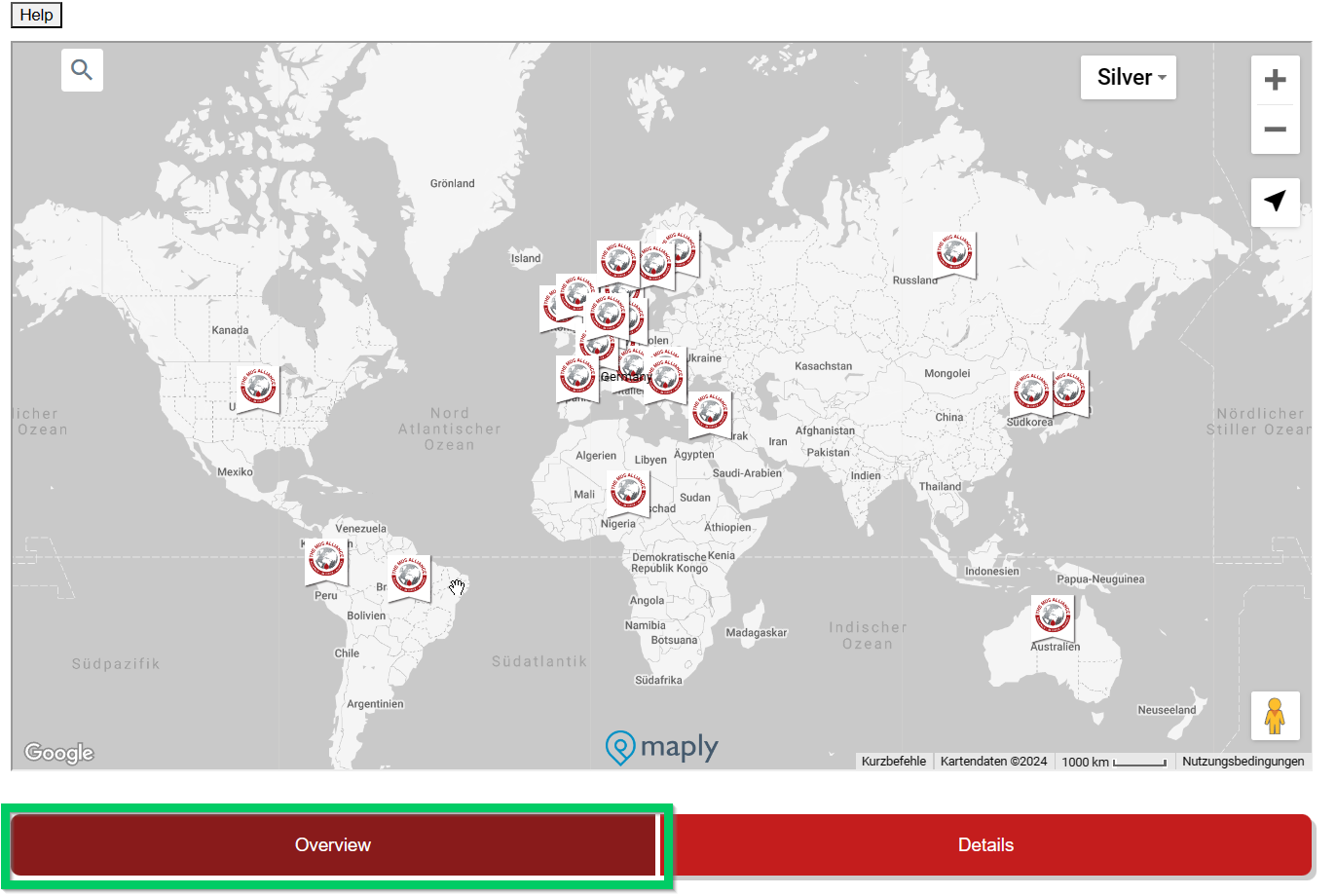
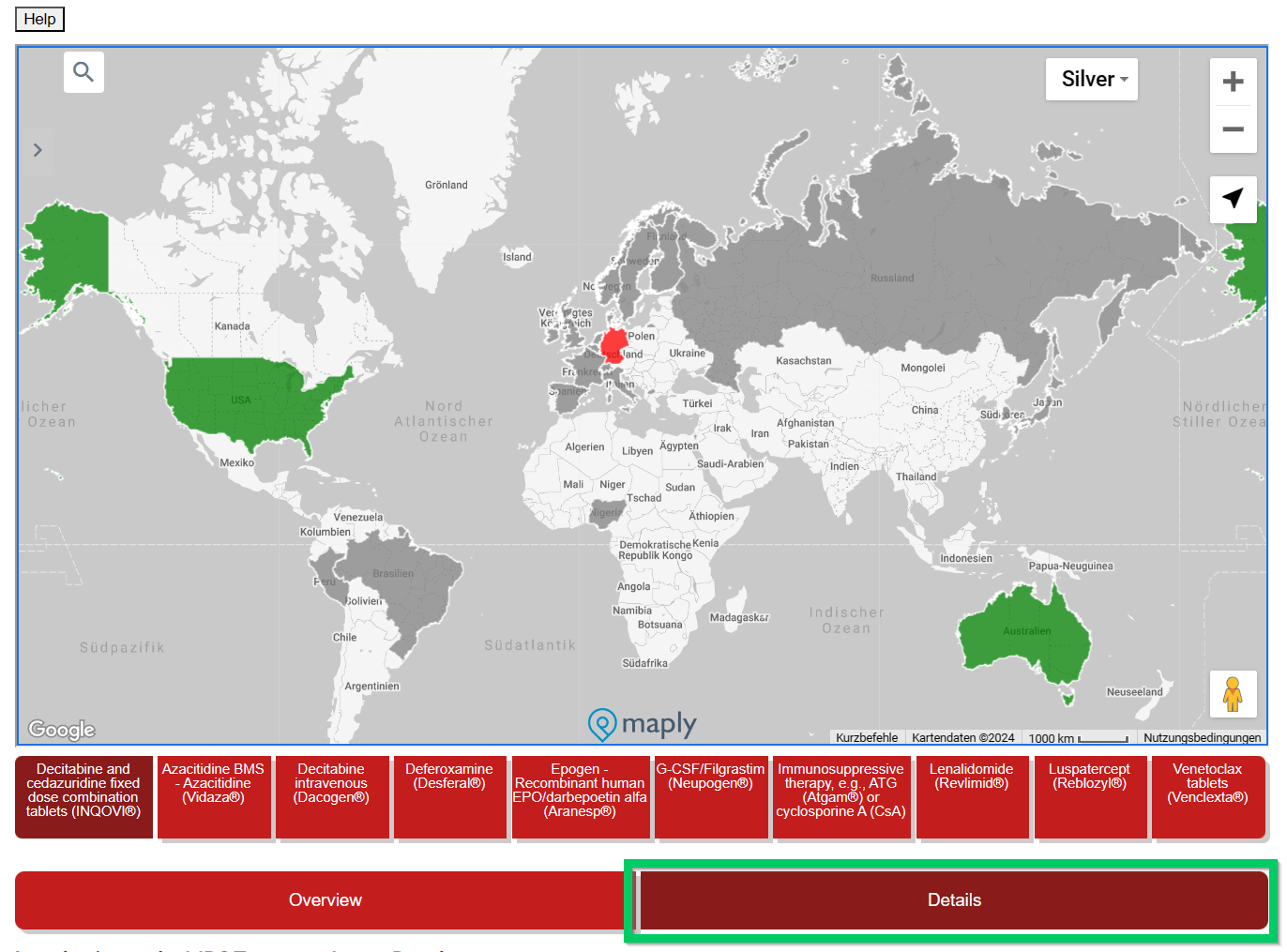

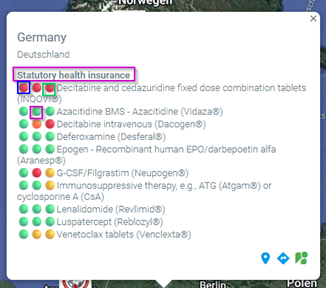 This overview is interactive and detailed information is displayed by moving the mouse. Under the country name there is a (pink) reference to the health insurance system (if known) in this country.
This overview is interactive and detailed information is displayed by moving the mouse. Under the country name there is a (pink) reference to the health insurance system (if known) in this country.