Update on the work around the ERN European Reference Networks.
Thursday, July 28, 2016
The 21st Congress of the European Hematology Association ( June 9-12, 2016) was abuzz with networking between over 10.000 participants who travelled from all over the world to Copenhagen. The EHA Congress combined sessions and a diverse range of topics around Hematology highlighting state-of-the-art clinical practice, recent advances, new data and views from different stakeholders and international organizations. A hot topic this year were the European Reference Networks.
European Reference Networks, a unique opportunity to take collaboration and patient care in hematology to the next level, was a core topic at EHA 2016
The 21st Congress of the European Hematology Association ( June 9-12, 2016) was abuzz with networking between over 10.000 participants who travelled from all over the world to Copenhagen. The EHA Congress combined sessions and a diverse range of topics around Hematology highlighting state-of-the-art clinical practice, recent advances, new data and views from different stakeholders and international organizations. A hot topic this year were the European Reference Networks.
EuroBloodNet: A European Reference Network for rare haematological diseases
On Saturday 11 June, a session in the Patient Advocacy Track focused on the emerging European Reference Networks (ERNs). Discussion on ERNs was a very timely occasion to exchange about the initial call and upcoming deadline (21 June 2016) for applications from networks of Centres of Expertise and healthcare providers wanting to become ERNs.
Before the year end, ERNs will be created as legal entities by the European Union, and will provide for the first time a unique opportunity to work cross border in Europe in healthcare where expertise is scarce. The core of these networks will be to develop and manage cohorts and registries, best practices of diagnostic and care and clinical trials.
On facilitation of the European Hematology Association, Prof. Pierre Fenaux from the Hopital Saint Louis in Paris is coordinating the rare hematology diseases ERN (EuroBloodNet) application together with Prof. Béatrice Gulbis from the University Hospital Erasme in Belgium, gathering highly specialized hospital centres of expertise of malignant and non-malignant rare hematological disease-specific clinical networks. Currently,ERN applications which were submitted by 21 June 2016 are being evaluated and will have to pass three steps – the eligibility check by the Commission and the independent assessment bodies, the technical assessment by the independent assessment bodies and the approval by the Board of Member States.
The EHA Patient Advocacy track included a Patient Advocacy Session on “Collaboration in networks to improve treatment and care: European Reference Networks”, chaired by Sophie Wintrich from the MDS Alliance and Jan Geissler from the CML Advocates Network. Speakers were Enrique Terol from the European Commission, the previous rare hematology ERN (EuroBloodNet ERN) coordinator Joan-Lluis Vives Corrons, Ananda Plate from Myeloma Patients Europe (MPE) and Till Voigtländer from the Board of Member States. The session was followed by a EHA Patient Fellows Capacity Building Training on “Patient involvement in European Reference Networks and in the hematology ERN” run by Matt Johnson from EURORDIS and focusing on the European Patient Advocacy Groups (ePAGs) with participation from EuroBloodNet ERN coordinator Prof. Fenaux. Breakout sessions then provided members with an opportunity to ask questions on ERNs, on the process for first call for ERNs and on how their patient organisation can be part of the ERN. The track ended with a Interview about EuroBloodNet.
Enhancing patient engagement in European Reference Networks: The European Patient Advocacy Groups (ePAGs)
Patients are represented and active participants in the ERN development process via the European Patient Advocacy Groups. Each ePAG corresponds to one of the 22 ERN groupings and brings together elected patient representatives and patient organisations who will ensure that the patient voice is heard in the development, programming and evaluation of ERN initiatives and activities. Today, 86 patient representatives are elected in these groups.
In May 2016, the haematology patient community elected five representatives into the rare hematology ePAG: Amanda Bok (European Haemophilia Consortium), Angelo Loris Brunetta (Associazione Ligure Thalassemici Onlus), Jan Geissler (Leukemia Patient Advocates Foundation), Ananda Plate (Myeloma Patients Europe) and Sophie Wintrich (MDS UK Patient Support Group) have actively participated in the development of the EuroBloodNet application over the last weeks and successfully ensured that the patient voice is fully represented in the ERN Board and sub-clinical committees – a great achievement and a milestone in increasing the role of patients in clinical care as it evolves in Europe.
It is important that the patient communities involved in ERNs grow and that patient representatives and clinicians evolve how they work together in the newly established system of ERNs. If you are representing a patient organisation and are interested in joining an ePAG to be involved and kept informed about ERN activities and to ensure they successfully meet the needs of patients across Europe, please get in touch with the ePAG representatives at haemepag@cmladvocates.net.
There is no limit on the number of member organisations of an ePAG. The movement as a whole can only benefit from the flow of ideas between associations and from the enriched debates and stronger sense of community with each ePAG.
What’s next?
The application for EuroBloodNet has been submitted by the application deadline on 21 June 2016. It is estimated that decisions on which ERN applications have been successful will be made by the end of the year, so that the implementation can start in early 2017. The ePAGs will follow the process, and will keep the community updated on news.
What are European Reference Networks (ERNs)?
European Reference Networks (ERNs) are platforms for clinicians and researchers to share expertise, knowledge and resources across the EU. This initiative of the European Commission, supported by all Member States, aims to address common challenges faced by professionals when diagnosing and providing highly specialised healthcare in complex, rare or low prevalence diseases. It does not interfere with already existing networks. ERNs will improve clinical outcomes and quality of life of people living with these type of conditions across the EU. ERNs are part of the legal framework of the EU Patients’ Rights in Cross Border Healthcare Directive. Funding will be provided by Member States and the European Commission to run these networks. EURORDIS has worked hard that patient involvement will be a key element of ERNs and their governance, and this has been adopted by the European Commission Expert Group for Rare Diseases. Hematology and Rare Cancers are two of the 22 disease groupings where the Commission and Member States have encouraged applications for ERNs.
More information on the EC website: http://ec.europa.eu/health/ern/implementation/call/index_en.htm
List of ERN applications: http://www.rd-action.eu/european-reference-networks-erns/coordination-of-rare-disease-erns/
To view and download this news release Click Here







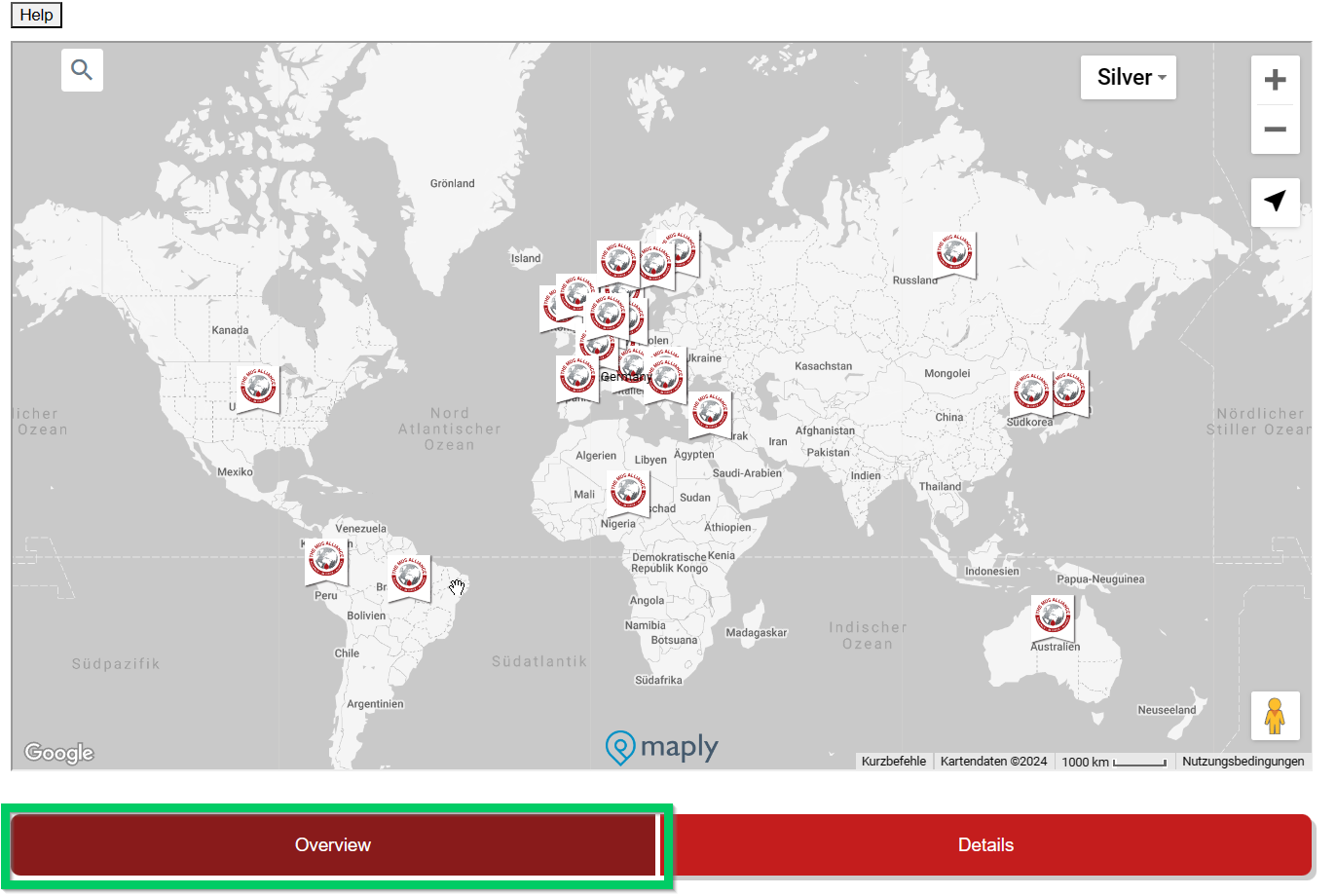
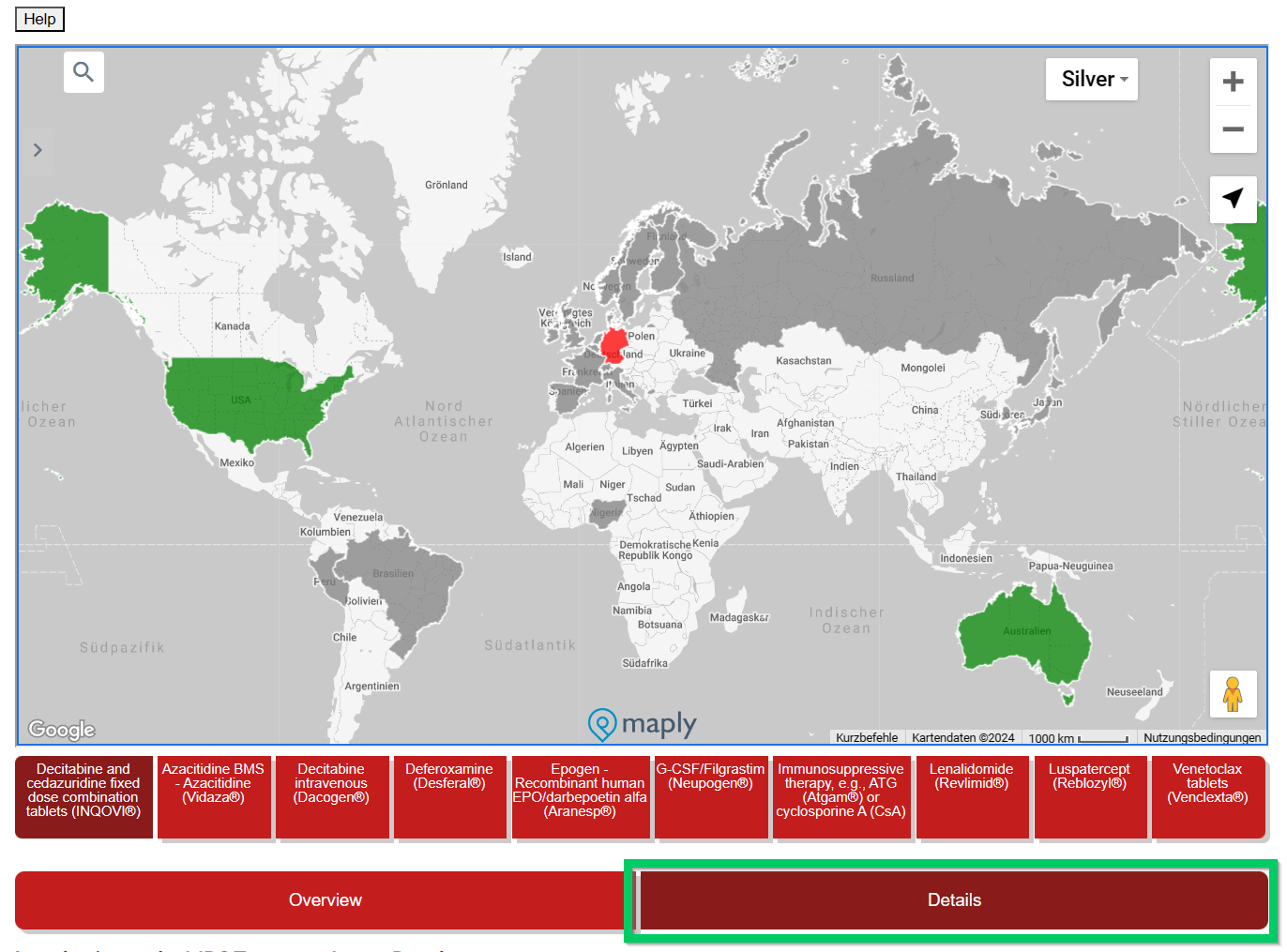

 This overview is interactive and detailed information is displayed by moving the mouse. Under the country name there is a (pink) reference to the health insurance system (if known) in this country.
This overview is interactive and detailed information is displayed by moving the mouse. Under the country name there is a (pink) reference to the health insurance system (if known) in this country.