15th, October, 2020
The HARMONY Alliance launches HARMONY PLUS, a new public-private partnership to improve outcomes for patients with blood cancers
- HARMONY PLUS builds upon the success of HARMONY in capitalizing on Big Data to speed up the development of more effective treatments for patients with blood cancers.
- It will leverage the HARMONY Big Data Platform, a central data pool of anonymized data, collected from public and private organizations. Real World Data will be added to the platform to perform Real World Evidence research projects.
- It will use data from the HARMONY Big Data Platform as a virtual control arm to support clinical trial design in the hematologic malignancies field.
- It will expand the focus of HARMONY to additional blood diseases and apply new Artificial Intelligence techniques.
HARMONY has made rapid progress since its launch in January 2017. By the end of 2019, HARMONY had identified 45,000 data sets from patients with various types of blood cancer and is targeting a data pool of 100,000 patients during the lifetime of the project. Furthermore, the HARMONY Big Data Platform represents a unique resource, meeting the highest standards of data security and governance.
Which blood cancers are targeted by HARMONY and which by HARMONY PLUS?
HARMONY focusses on Acute Lymphoblastic Leukemia (ALL), Acute Myeloid Leukemia (AML), Chronic Lymphocytic Leukemia (CLL), Multiple Myeloma (MM), Myelodysplastic Syndromes (MDS), Non-Hodgkin Lymphoma (NHL) and Pediatric Hematologic Malignancies.
HARMONY PLUS is widening the scope of HARMONY to cover myeloproliferative (MPN) disorders and other hematological malignancies. The new targets are Chronic Myeloid Leukemia (CML), Polycythaemia Vera (PV), Essential Thrombocythemia (ET), Myelofibrosis; Hodgkin’s Lymphoma, Waldenström Macroglobulinaemia and other rare blood cancers.
How are patients involved?
Patients are a fundamental source of real-world data, but their contribution and specific expertise has long been a secondary priority in discussions about real-world evidence (RWE). HARMONY PLUS will provide an increased focus on RWE and the involvement of patient advocacy organisations.
The HARMONY Patient Cluster, a unique group of seven pan-European patient umbrella organizations which are active in HARMONY’s haematological malignancies, was an integral part of the HARMONY Alliance right from the start.
The patient community has actively contributed e.g. to the review of Big Data research proposals, the definition of Core Outcome Sets, and the project’s ethics and data anonymisation procedures.
Within its broadened disease scope, HARMONY PLUS will expand its Stakeholders and Patients’ Organisations Forum to enable additional pan-European patient organizations as well as nurses organisations to contribute and co-create in the different workstreams of HARMONY PLUS.
—
18th July, 2019
How will the Harmony Project lead to a more personalised and targeted treatment?
The Harmony Project has produced 3 excellent videos that explain how the Harmony research will lead to more personalised and targeted treatment.
Please make use of them to explain the project to patients in your own country.
In the absence of game changer drug for the treatment of MDS, the Harmony Project offers the reassurance that the response rates of current treatments will improve, by selecting a treatment for individual patients that is more likely to work by matching their disease to a specific sub-group.
Watch this video to learn how Big Data can help clinicians to treat blood cancer
By studying big amounts of data, researchers will be able to predict the development of a particular disease and how certain subgroups of patients will respond to treatment.
This should result in tools that will enable clinicians and doctors to rapidly select the most promising treatment for a particular patient.
28th April, 2019
Harmon-ising with the HARMONY Project
Attended by Blerina Ahmetaj-Shala (Kosovo Group) and Mirjami Tran Minh (Finland Group) at the EHA Offices in The Netherlands on 4th February 2019
Although we had heard the word HARMONY splattered around a few times we were not really sure what it was. This is why we jumped at the opportunity to attend the HARMONY Project session at the EHA office in The Hague, The Netherlands on 4th February 2019.
It was a pretty full on session beginning at 10.30am and finishing at 18:30pm. Despite the long hours, the time flew quickly and there were sufficient opportunities to network over break and lunch. People present in the workshop included the HARMONY Patient Cluster representatives (a unique group of 7 European Patient Umbrella Organization including MDS Alliance; at least two from each organisation), pharmaceutical company representatives and EHA employers.
What is HARMONY?
After a brief introductory session from Ellen de Waal, the communications manager for HARMONY Alliance, Mirko Vujkevic who is the Novartis HARMONY Project lead gave an interesting update on the topic. HARMONY Project, otherwise named as ‘Big data for better outcomes’, is a high quality big data platform, which aims to speed up drug development, access and pathways to drugs and bench-to-bench side practice. It objectives are to increase harmonisation of outcome measures and endpoint definitions and increase the application of omics data in clinical practice.
With this in mind, there are four key domains of a patient centric approach in HARMONY; (1) patient partnership, (2) transparency to patients (3) inclusiveness of patients and (4) patient centered data sources.
Currently HARMONY Alliance (https://www.harmony-alliance.eu/en/about-us) consists of 53 partners and 32 Associated Members from 22 countries, including 8 pharmaceutical companies from the European Federation of Pharmaceutical Industries and Associations (EFPIA).
What is included in the HARMONY Project?
Ana Heredia Casanoves discussed what is included in the ‘Big Data Platform’. Parameters for testing include treatment, quality of life, omics data, diagnosis, resources and demographics. The different sections/data that people can access are based on the research proposal that was provided. Only sections that are necessary to answer the research will be accessible. There will a constant audit trail and various firewalls in place to ensure complete security.
How can we apply for grants?
If you have an unmet need that only HARMONY can help answer then you should get in touch with HARMONY Alliance. Patient groups can propose a research project based on unmet medical needs, which Harmony may take as part of their research programme in collaboration with the patient organisation. If you need more information, then the link to the Community Forum is: https://internal.harmony-alliance.eu/. If you do not have a profile yet, you can create one – approval will be granted asap
Other interesting points
At this session I also got to learn about other tools such as the EHA Campus. This excellent education platform gives you access to learning tools created for and by hematology professionals, packed with features especially designed for learners. The course is available for doctors to recognise lab results and tests their knowledge. All parts of the course are based on European Hematology guidelines with the aim of bringing all those that take part to the same basic knowledge level. In addition to this if you register and login to the HARMONY Alliance website, you can have access to many different resources including excellent videos, brochures and a conversation forum where you can connect with other alliance partners and associate members regarding topics of interest or questions that you have.
Final remarks…
Overall this was an excellent introductory session to the HARMONY Project voiced directly by the people developing the platform. We, as patient representatives were actively encouraged to ask questions, and also discussed potential research ideas that we could put forward in our specific disease areas. It is clear that despite a slow start, progress is being made in making this an innovative platform that can increase output by enabling enrichment and unlocking new insights into hematological diseases.
13th March, 2019
Moving Forward: First Data Transferred Into The HARMONY Big Data Platform
An important step in search of new methods of treatment of Hematological Malignancies: HARMONY Partners Ulm University, Novartis and Erasmus University Medical Center uploaded research data into the HARMONY Big Data Platform.
More Partners and Associated Members announce their readiness to contribute data from their sources. The HARMONY Alliance is a large public-private European Network of Excellence for Big Data in Hematology, established in January 2017
and funded by the Innovative Medicines Initiative (IMI).
Although the cytogenetic heterogeneity of AML has been recognized for many decades, the molecular heterogeneity of the disease has been identified only over the past 15 years. The prognostic value of the heterogeneity is widely accepted. However, translation of this molecular information into improved therapeutic regimes is slow.
In its first pilot project, HARMONY is combining patient information from the German Austrian AML Study Group (AMLSG) and the HOVON – the Haemato Oncology Foundation for Adults in the Netherlands- registries with the aim to further improve disease classification and better understand the genomic context, to identify other prognostic factors and to monitor outcomes with current treatment and new therapies in different subtypes of adult AML.
In this context, Novartis has provided anonymized data from the RATIFY trial.
“HARMONY will become an invaluable tool to better understand the heterogeneity of hematologic malignancies and to move big data analysis to the next level”, says Hartmut Döhner from HARMONY Partner Ulm University Hospital and chair of the German-Austrian AML Study Group (AMLSG). Ulm University Hospital provided 1540 datasets.
Novartis shared data from the Rydapt (midostaurin) RATIFY study, the largest clinical trial in FLT3-mutated AML to date, performed to determine the effect of adding midostaurin, a multitargeted kinase inhibitor, to standard chemotherapy in patients with AML and a FLT3 mutation. This data, which was anonymized before transfer, will be integrated and analyzed along with data from a number of other high-quality sources within HARMONY, to help define clinical endpoints and outcomes for AML. The Phase III RATIFY trial was run in collaboration with the Alliance for Clinical Trials in Oncology (CALGB). It included 717 study participants from around the world. The full results, which showed significant overall survival benefit for FLT3+ AML patients consistently across FLT3 mutation subgroups, were published in the New England Journal of Medicine in June 2017 under the senior authorship of the HARMONY Key Opinion Leader Hartmut Döhner (ref Stone RM et al. N Engl J Med. 2017 Aug 3;377(5):454-464). Based on data from RATIFY, The National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for AML now include the use of midostaurin in FLT3-mutated AML.
“With such positive efforts in sharing aggregated data of hundreds of patients into the HARMONY platform, the hope is that other data custodians across industry and academia will also be inspired to join the HARMONY Alliance. In the future, the aspiration is that the HARMONY Alliance model will allow us to create a blueprint that can be applied to future projects in other disease areas, ultimately benefiting many more patients in Europe and well beyond”, states Mirko Vukcevic from Novartis and HARMONY Project Lead.
Other datasets (680) come from the Erasmus University Medical Center in Rotterdam. The department of Hematology has been engaged in the collection of genomics data sets of clinically well-annotated patients enrolled in the international multicenter HOVON-SAKK AML trials. This way they will contribute the published data sets – which include gene expression data (Affy), copy number data (Affy), and targeted NGS data of AML – to HARMONY.
“This data will give better insight into the heterogeneous disease AML”, adds Dr. Peter Valk from Erasmus MC, HARMONY Partner, who focuses in his research on the molecular analyses of various hematologic malignancies, mainly acute myeloid and lymphoblastic leukemias.
In the next generation sequencing era, large data sets of (subset of) acute myeloid and lymphoid leukemias of patients enrolled in the HOVON-SAKK clinical trials will be generated. These data sets currently comprise targeted gene panels on AML cases at diagnosis and after high dose induction treatment but will also include whole exome and whole genome data in the future. They will also be contributed to the HARMONY Alliance. The first three data sources are just the beginning. More HARMONY Partners and Associated Members are getting ready to upload their anonymized data from clinical trials or other research projects to the HARMONY Big Data Platform. Furthermore, it is not a one-off process with a specified end. Big Data analysis will be carried out over a long period of time. Alongside the gradually added and upgraded data sets and rising amounts of information, a data mining process will enable us to make new discoveries in the connected databases.
The HARMONY Big Data Platform: Algorithms to transform knowledge into better medicine outcomes for hematology patients.
Read more >
The HARMONY Anonymization Concept reconciles data quality, safety, and privacy.
—-
London – November 23/24, 2017
On 23 and 24 November 2017 the HARMONY Alliance organized a workshop on the definition of meaningful outcomes for hematological malignancies.
We were invited as patients advocates, together with 60 stakeholders to discuss outcomes relevant to all stakeholders across all haematological malignancies.
Brussels/Salamanca – January 9, 2017
The Innovative Medicines Initiative (IMI) has approved HARMONY, a project that aims to foster better access and care for patients with various hematologic malignancies (HM) with the use of big data. The project is made up of 51 partners from 11 European countries, including 7 pharmaceutical companies.
Other organisations such as Myeloma Patients Europe (MPE), Lymphoma Coalition, MDS Alliance and CLL Advocate will be involved in this project as well in order to provide the patient perspective.
MDS Alliance will attend the kick-off meeting in mid-January 2017, together with the other patient advocacy groups and all stake-holders.
More news to follow soon.
PRESS RELEASE
INNOVATIVE MEDICINES INITIATIVE APPROVES
€ 40 million PROJECT FOR BETTER CARE OF PATIENTS WITH HEMATOLOGIC MALIGNANCIES
Brussels/Salamanca, January 9, 2017. The Innovative Medicines Initiative (IMI) has approved HARMONY, a project that aims to foster better access and care for patients with various hematologic malignancies (HM) with the use of big data. The project is made up of 51 partners from 11 European countries, including 7 pharmaceutical companies.
HARMONY will capture, integrate, analyze and harmonize anonymous patient data from high-quality multidisciplinary sources to unlock valuable knowledge on multiple myeloma (MM), acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), non-Hodgkins lymphoma (NHL), myelodysplastic syndromes (MDS) and pediatric HMs.
Building on pre-existing, long-lasting collaborations between Academic institutes and the pharmaceutical industry, the HARMONY project shall further advance HM management through a more efficient process of treatment development and rapid decision-making. The expected outcome will be better prognosis and quicker life-saving decisions, important for patients suffering from these hematological diseases.
The project brings together key stakeholders in the clinical, academic, patient, HTA (health technology assessment), regulatory, economical, ethical and pharmaceutical fields to:
- Developing a data sharing platform that empowers clinicians and policy stakeholders to improve decision-making
- Establishing a network reflecting the European HMs landscape
- Defining clinical endpoints and standard outcomes in ALL (paediatric & adult), NHL, MM, AML, CLL, MDS
- Alignment of key stakeholders on relevance of these outcomes (policy makers, payers, patients)
- Providing means for analysing complex data sets comprising different layers of information
- Identifying specific markers for early registration of innovative and effective therapies for HMs
The HARMONY project’s final deliverable is a big data platform which integrates outcome measures and endpoint definitions for HMs. HARMONY will achieve this from a pan-European perspective by uniting and aligning European stakeholders and key opinion leaders in the field. The 5-year project will start in January 2017 and is funded through the Innovative Medicines Initiative (IMI); Europe’s largest public-private initiative aiming to speed up the development of better and safer medicines for patients. Harmony is coordinated by two public leads: Prof. Dr. Jesús Marïa Hernández from Instituto de Investigación Biomédica de Salamanca, Spain, and Dr. Guillermo Sanz from Instituto de Investigación Sanitaria del Hospital La Fe de Valencia, Spain, and by two EFPIA leads, Tayyab Salimullah from Novartis Oncology and Pam Bacon from Celgene International.
Contact information: Harmonyoffice@ibsal.es

THE INNOVATIVE MEDICINES INITIATIVE
The Innovative Medicines Initiative (IMI) is Europe’s largest public-private initiative aiming to speed up the development of better and safer medicines for patients. IMI supports collaborative research projects and builds networks of industrial and academic experts in order to boost pharmaceutical innovation in Europe. IMI is a joint undertaking between the European Union and the pharmaceutical industry association EFPIA.
Disclaimer
It should be made clear in the text and layout that the communication reflects the author’s view and that neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained therein.
Follow us on: Facebook | LinkedIn | Twitter
Partners in HARMONY
Project management
- Instituto de Investigación Biomédica de Salamanca (IBSAL), Spain
- Instituto de Investigación Sanitaria del Hospital La Fe de Valencia (HULAFE), Spain
- Celgene International II SARL, Switzerland
- Novartis, Switzerland
- Synapse Research Management Partners, S.L. (SYNAPSE), Spain
Partners
- Universitaet Ulm (UULM), Germany*
- Alma Mater Studiorum – Università di Bologna (UNIBO), Italy*
- European Hematology Association (EHA), The Netherlands*
- ELN Foundation (ELN), Germany*
- GMV Soluciones Globales Internet S.A.U. (GMV), Spain*
- European Alliance for Personalised Medicine (EAPM), Belgium
- Medizinische Universitaet – Wien (MUW), Austria*
- Erasmus Universiteit Medisch Centrum Rotterdam (EMC), The Netherlands
- University of Navarra (UNAV), Spain
- Università degli Studi di Torino (UNITO), Italy
- Stichting VUmc, The Netherlands
- The Chancellor, Masters and Scholars of the University of Cambridge (UCAM), United Kingdom
- University of Rome ‘Tor Vergata’ (URTV), Italy
- Goethe University Frankfurt (GUF), Germany
- Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL), France
- Jose Carreras Leukaemia Research Institute (IJC), Spain
- European Research Initiative on CLL e.v. (ERIC), Germany
- Masaryk University (MU), Czech Republic
- Fundacio privada Institut D’investigacio Oncologica de Vall-Hebron (VHIO), Spain
- The Lymphoma Scientific Association (LYSA), France
- Ludwig-Maximilians-Universitaet – Muenchen (LMU-Muenchen), Germany
- Barts Health NHS Trust (BHT), United Kingdom
- Groupe Francophone des Myélodysplasies (GFM), France
- Heinrich-Heine-Universitaet – Duesseldorf (UDUS), Germany
- Fondazione Italiana per lo studio delle sindromi mielodisplastiche onlus (FISMonlus), Italy
- University of Newcastle upon Tyne (UNEW), United Kingdom
- German Society of Pediatric Oncology-Hematology GmbH gemeinnützig (GPOH), Germany
- Ospedale Pediatrico Bambino Gesù (OPBG), Italy
- University of York (UoY), United Kingdom
- European Organisation for Research and Treatment of Cancer (EORTC), Belgium
- European Society for Blood and Marrow Transplantation (EBMT), The Netherlands
- Flanders Institute of Biotechnology (VIB), Belgium
- University of Helsinki (UH), Finland
- Assistance Publique – Hôpitaux de Paris (AP-HP), France
- Genome Research Limited (GRL-SANGER), United Kingdom
- MediSapiens Ltd (MS), Finland
- MLL Munich Leukemia Laboratory GmbH (MLL), Germany
- LeukaNET (LeNET), Germany*
- National Institute for Health and Care Excellence (NICE), United Kingdom
- Agencia Española de Medicamentos y Productos Sanitarios (AEMPS), Spain
- Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM), Germany
- Amgen, United Kingdom*
- Janssen Pharmaceutica NV, Belgium*
- Bayer Aktiengesellschaft, Germany*
- Menarini Ricerche S.p.A., Italy*
- Takeda, United Kingdom*
*Work package leaders and co-leaders










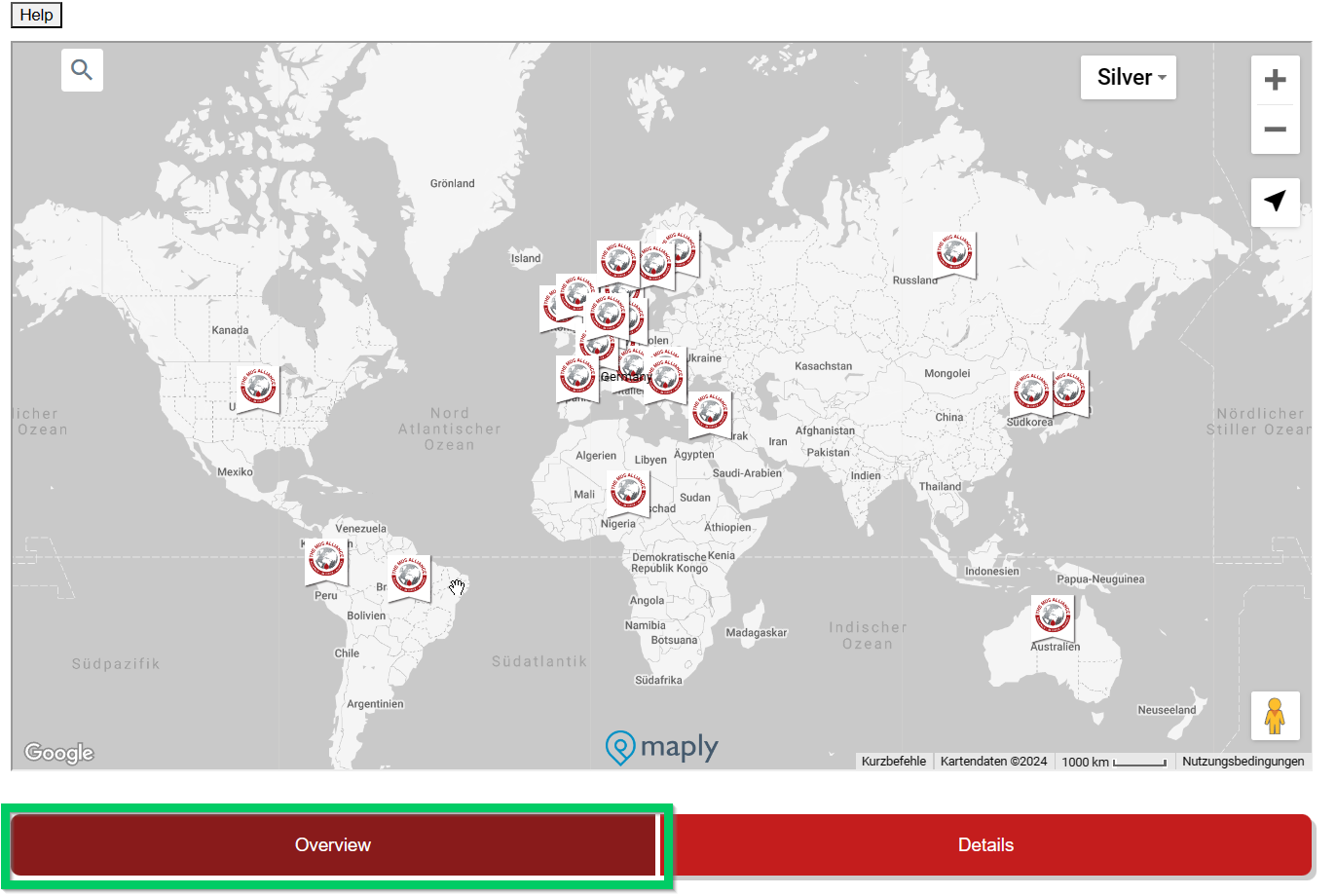
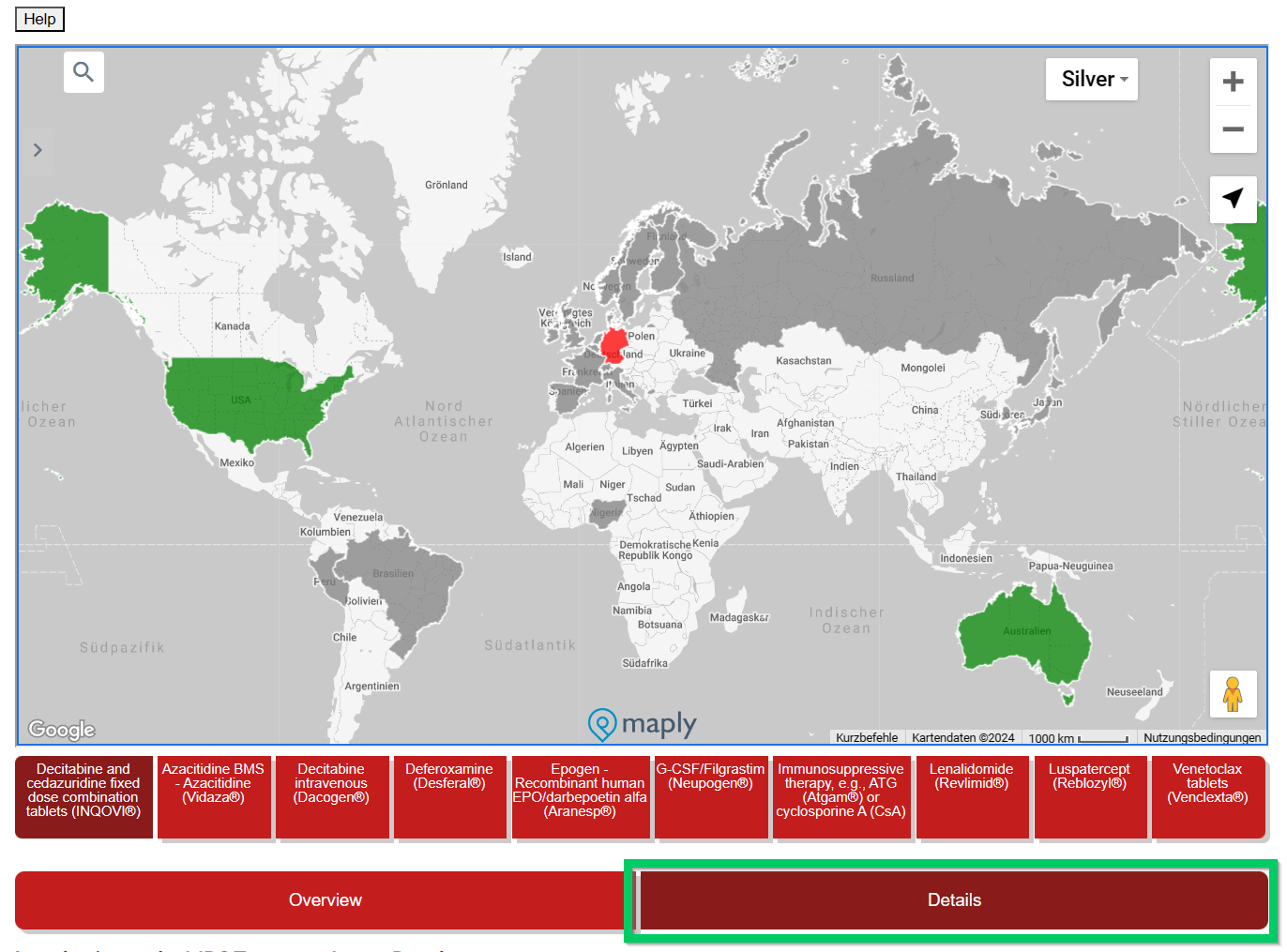

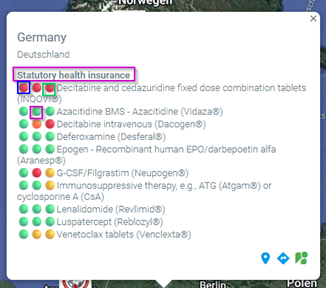 This overview is interactive and detailed information is displayed by moving the mouse. Under the country name there is a (pink) reference to the health insurance system (if known) in this country.
This overview is interactive and detailed information is displayed by moving the mouse. Under the country name there is a (pink) reference to the health insurance system (if known) in this country.